Pharma & Cosmetics
Anti-Counterfeiting & Traceability

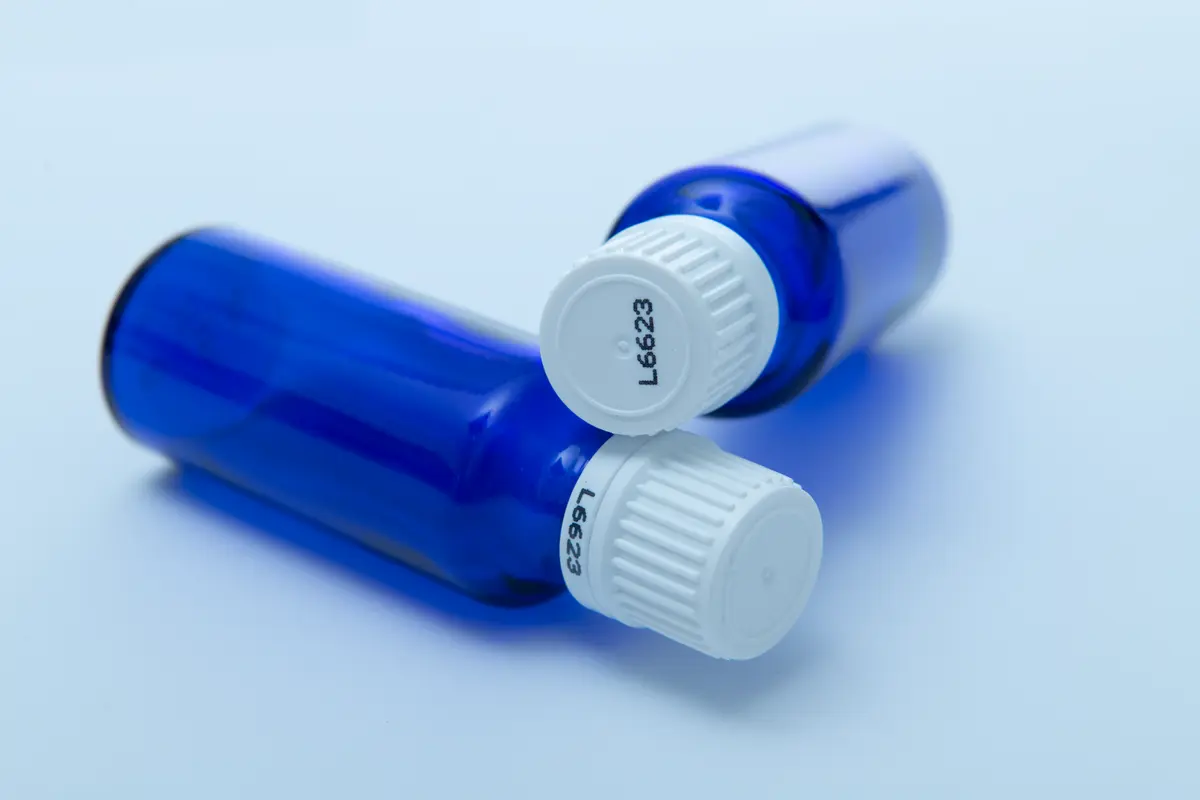
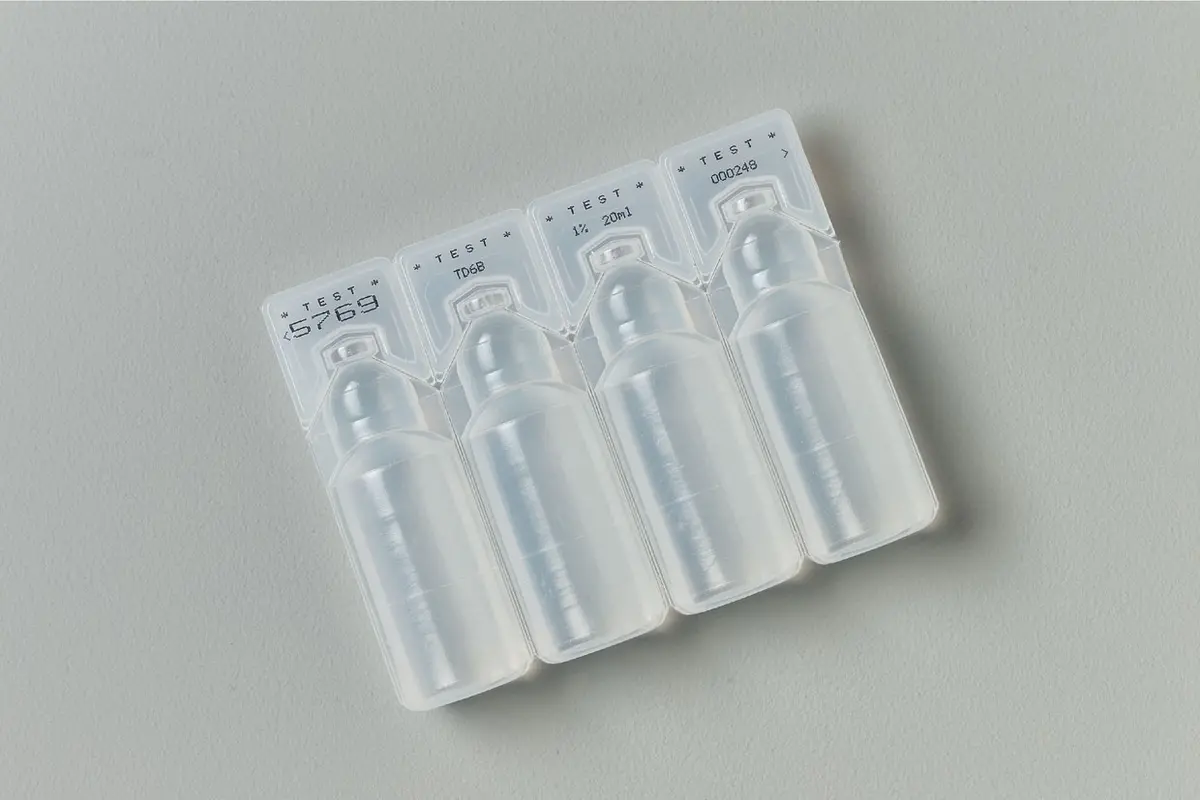
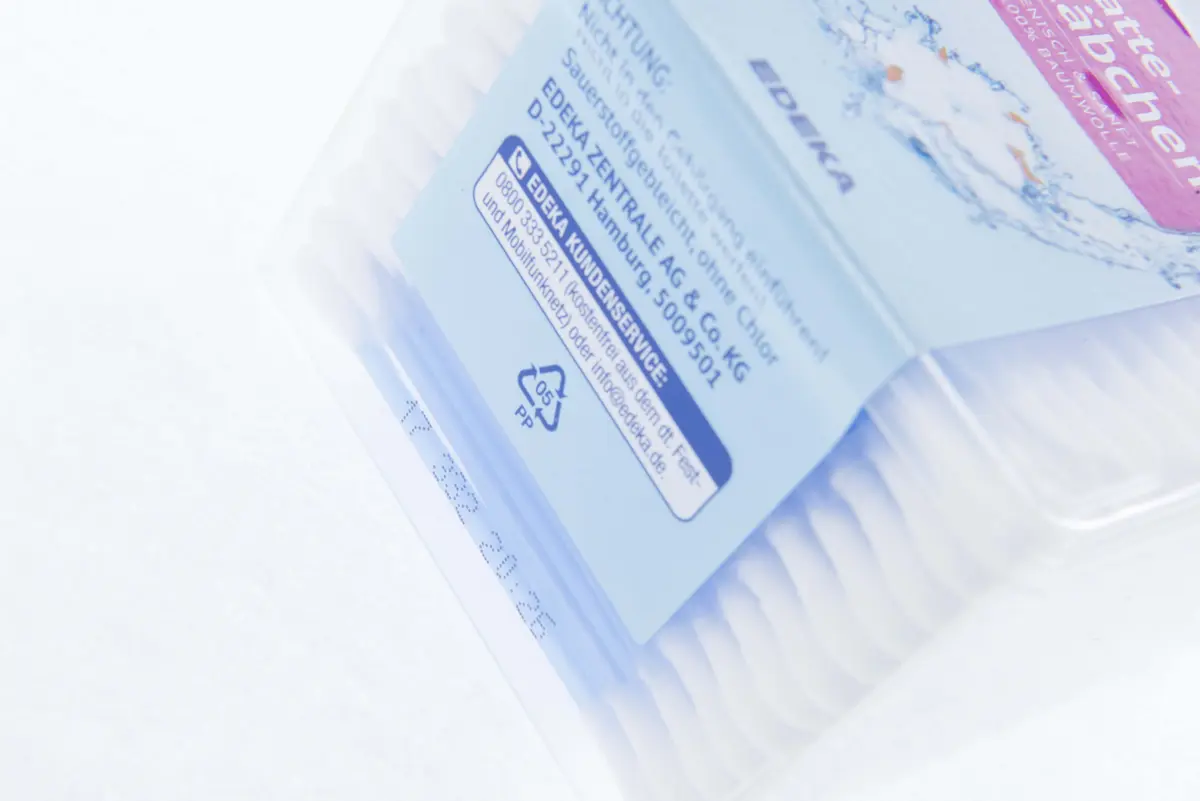
Marking on Pharmaceuticals & Cosmetics
In the pharmaceutical and cosmetics industries, trust, quality, and safety are essential. Regulations such as the Falsified Medicines Directive (FMD) and Good Manufacturing Practice (GMP) demand precise systems that integrate seamlessly into existing production and supply chains.
Koenig & Bauer Coding delivers cutting-edge solutions for anti-counterfeiting, end-to-end traceability, and maximum production security.
Precision and innovation that protect your brand – reliably and efficiently.

You benefit from...
Product Safety
By combining robust authentication with precise coding, you create tamper-proof markings that guarantee the protection and authenticity of your pharmaceutical and cosmetic products. This not only builds customer trust but also ensures compliance with the highest regulatory standards.
Smart Integration
Our interface protocols ensure seamless integration of our systems into almost any production environment. And when things get more complex, our application experts are by your side from the initial concept all the way to final implementation—both on the hardware and software side.
Compliance
With our solutions, you meet all legal requirements, industry-specific standards, and international regulations, from GMP and FMD to GS1. Whether in pharmaceuticals, cosmetics, food, or industry, Koenig & Bauer Coding ensures you’re always on the safe side.
Creativity
Cosmetics and skincare products are expected to enhance beauty. Their packaging must reflect the high quality of the product and appeal to customers through both design and functionality. We offer solutions for aesthetically sophisticated labeling to showcase your brand perfectly.
Excellent Marking Results for Many Years
Where tradition meets innovation: “Kräuterhaus Sanct Bernhard KG”, a company synonymous for over 100 years with high-quality natural remedies and personal care products, relies on cutting-edge technology from Koenig & Bauer Coding for product marking. For many years, their proven hot stamping systems have delivered precise and efficient marking, impressing with reliability and first-class customer support. Managing Director and pharmacist Karl Löther especially values the dependability of these devices and the excellent service.
For their new high-end product line, Kräuterhaus Sanct Bernhard once again turned to the experts at Koenig & Bauer Coding, choosing the alphaJET mondo. This fast, cost-efficient inkjet printer stands out with its low consumption and delivers brilliant print results on a wide variety of surfaces. Effortlessly meeting the company’s high standards, the alphaJET mondo ensures a powerful and reliable marking solution that is well-equipped to handle future production demands. All while providing top-notch print quality.

Looking for the right coding and marking solution?
Request more information now – we’re happy to help you find the right fit for your application.
Matching Technologies

Continuous-Inkjet Systems

Laser Systems

DOD-Inkjet Systems
High Resolution Print Quality
Drop-on-demand (DOD) for detailed and future-proof print results

Print & Apply Systems

Hot-Foil Systems

Thermal-Transfer Systems

Offline Coding Solutions
Efficient, Independent and Individual
Flexible marking outside of production for small and medium-sized batches
Read more
FAQs - Your Questions, Our Pharma & Cosmetic Answers
Pharmaceutical marking refers to the detailed information displayed on the packaging or containers of medicinal products. It encompasses all written or graphical content designed to inform healthcare providers, patients, and regulatory authorities about the drug’s properties, usage, and safety. These labels ensure the medication is accurately identified and used correctly by providing clear instructions for dosage, administration, and storage. Pharmaceutical labels also play a critical role in communicating potential risks, precautions, and contraindications. They are essential for ensuring compliance with legal and regulatory standards while safeguarding patient health through proper communication of key drug details.
Pharma coding and marking is a requirement for a wide range of medicinal products, including prescription drugs, over-the-counter (OTC) medications, and certain supplements classified as pharmaceuticals. Items such as injectable medications, tablets, capsules, syrups, and topical formulations must carry appropriate labeling. In addition, medical devices, diagnostic agents, and any products containing active pharmaceutical ingredients (APIs) typically require labeling that adheres to strict industry and regulatory standards. These labels ensure traceability, compliance, and the provision of vital information for safe use, making them indispensable for all medicinal products reaching consumers or healthcare facilities.
Pharmaceutical labels must include a range of mandatory details to ensure safety, compliance, and effective use of the medication. Key elements typically include the drug’s name, active ingredients, strength, and dosage form, along with specific instructions for use and storage. Additional information may highlight warnings, potential side effects, and contraindications, helping to minimize risks during usage. Labels also often include batch numbers, expiration dates, and manufacturer details to ensure traceability and accountability. Depending on the jurisdiction, certain labels may also require serialization codes for tracking and anti-counterfeiting purposes, particularly in regulated markets like the EU and the US.
Fields marked with a * are mandatory. You will receive an e-mail confirming your contact request after you have filled out this form.
 English
English
 Deutsch
Deutsch
 Français
Français
 Polski
Polski
 Dutch
Dutch
 Swedish
Swedish